Originally released by Organon, which has since merged with Merck & Co., NuvaRing is the original birth control ring released worldwide. However, many NuvaRing users have alleged that the devices have caused severe side effects, such as blood clots and other life-threatening health issues. Some users have died, leading their families to file NuvaRing lawsuits against Merck & Co., as well.
Scientific research has, indeed, shown that NuvaRing users have a higher risk of blood clotting injuries, and a higher risk of many side effects than the warning label informs them, including strokes, deep vein thrombosis, pulmonary embolism, and death. Over 1,983 lawsuits were combined into a federal multidistrict litigation, and 215 state lawsuits were combined into a multicounty litigation in New Jersey. As of 2019, there are still 89 cases pending.
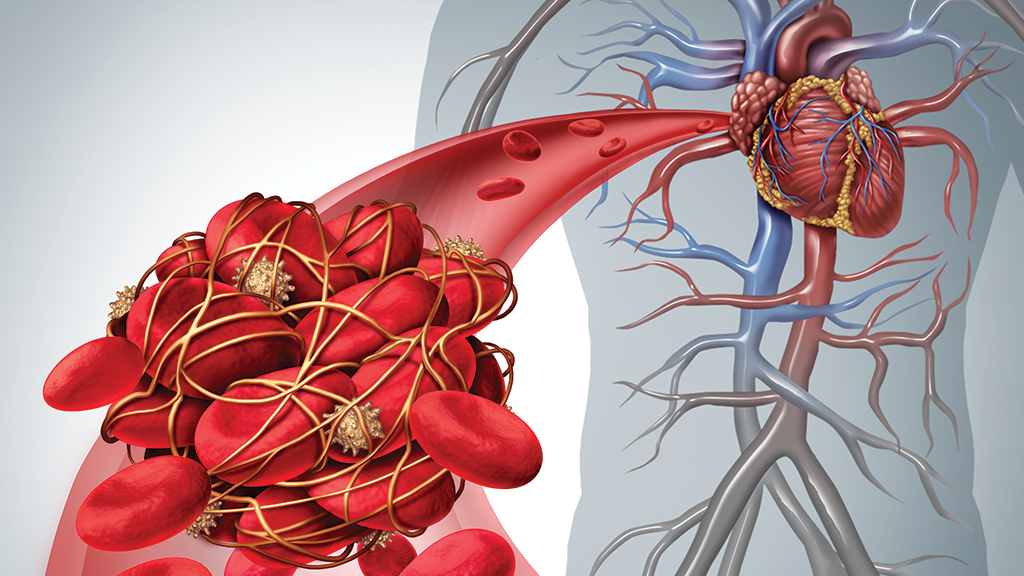
About NuvaRing
NuvaRing is a contraceptive vaginal ring that is placed into the vagina. Here, it is used to prevent pregnancy by releasing a low dose of etonogestrel (a progestin hormone) and an estrogen over a three week long cycle.
While many users have reported satisfactory use of NuvaRing, further research discovered that it came with a higher risk of dangerous complications than had been published on the warning labels. Furthermore, healthy women who made use of NuvaRing began to experience serious health issues, with venous thromboembolic events like blood clots in the legs and in the lungs being the most common.
Studies about the health risks of NuvaRing
An eight-year study was conducted from 2001 to 2007, working with over 800,000 hormonal contraceptive users, finding evidence of increased risk of blood clots. The FDA (US Food and Drug Administration, also noted the risk of embolisms as a significant safety concern before approving NuvaRing, but failed to publish the warning initially.
As of October 2013, the FDA required NuvaRing to publish warnings about the increased risk of embolisms and cardiovascular issues on the warning label. This point, several other studies had already been published, reporting on NuvaRing’s increased likelihood of such dangerous side effects compared to other hormone contraceptives, patches, and birth control pills.
The NuvaRing allegations against Merck & Co.
Many NuvaRing users allege that Merck & Co. was either negligent or knowingly deceitful, stating that the manufacturer should have known about the risk and done more to warn users about it. From 2007 onwards, more and more women and families began to come out with reports of serious medical complications, such as embolisms, clotting issues, and much more despite being healthy women prior to using NuvaRing.
In total, there were more than 2,100 NuvaRing lawsuits against Merck & Co, accusing the manufacturer of failing to test the effects of etonogestrel, the hormones in NuvaRing that has since been found to be the main risk factor. Furthermore, the lawsuits stated that the safety information published with NuvaRing was based on oral use, alone.
Health risks of NuvaRing
While the NuvaRing labels did come with health warnings, they primarily emphasised what users considered more minor side effects such as breast tenderness and nausea. However, many users went on to suffer from blood clots, strokes and heart attacks, high blood pressure, blood disease, and breast and reproductive organ cancer.
The NuvaRing lawsuits gained more attention following the death of 43-year old Ann Tompkins from Nebraska. After Tompkins died of a pulmonary embolism due to a blood clot in her lungs only five months after beginning to use NuvaRing, her estate filed a lawsuit in 2010. Ericka Langhart, a 24-year old woman also died of four massive heart attacks caused by a pulmonary embolism. Her doctors attested that they believed NuvaRing to be the cause and, in 2011, her parents filed another lawsuit against Merck.
Although the FDA published large-scale studies on the increased risk of blood clots associated with NuvaRing in 2011, and the high publicity of lawsuits against Merck & Co., there has been no NuvaRing recall.
NuvaRing Lawsuit Settlements
In 2014, Merck & Co. offered a settlement of $100 million to all cases, to be split between around 3,800 people, including some whoses claims had not yet been filed in court. Those who wished to enroll in the settlement were required to have an injury caused by NuvaRing before February 7, 2014, and had to submit an opt-in form and claim their portion before a final deadline of July 21, 2014.
The settlement of $100 million was split between all claimants, so not all recipients got around $60,000, which would be an equal share. Instead, the case was settled in tiers, so each claimant got a share of the money depending on the severity of their health issues related to NuvaRing.
However, several claimants, including the parents of Erika Langhart, the 24-year old woman who died of complications caused by a pulmonary embolism, decided not to opt into the settlement.
Are lawyers still taking NuvaRing lawsuit cases?
There are still NuvaRing cases pending against Merck & Co., but the majority of law firms are not taking on new NuvaRing lawsuits. This is due to the fact that, as of 2013, NuvaRing now has an updated label that describes the increased risk of blood clots and other related health issues. Merck & Co.’s settlement in 2014 was accepted by the majority of claimants, and all others who had suffered from NuvaRing related injuries before 2014 had officially missed the deadline if they had not opted in for settlement by July 21, 2014.